The Tootle Lab’s long-term objective is to understand how particular prostaglandin signaling events result in specific biological outcomes. Prostaglandins are locally acting, transient hormones that mediate a wide variety of biological activities, from female reproduction to cancer development and progression. Prostaglandins are synthesized at their sites of action by cyclooxygenase (COX) enzymes, the targets of Aspirin and Advil. As prostaglandin signaling is transient and locally acting, to determine the molecular mechanism of prostaglandin action a cell-cell signaling model is needed. Drosophila is an excellent system to use, as Drosophila genetics has been routinely employed to identify and characterize signaling cascades at single cell resolutions.
Previously, we developed Drosophila oogenesis as a new and powerful model for studying prostaglandin signaling. Using both pharmacology and genetics, we discovered that prostaglandins mediate Drosophila follicle development, identified the Drosophila COX1 enzyme, Pxt, and revealed that genetic perturbation of prostaglandin signaling can be used to exam the function of prostaglandins. This research reveals that prostaglandin signaling modulates actin/membrane dynamics, cell migration, stem cell activity, and the timing of gene expression during Drosophila follicle development.
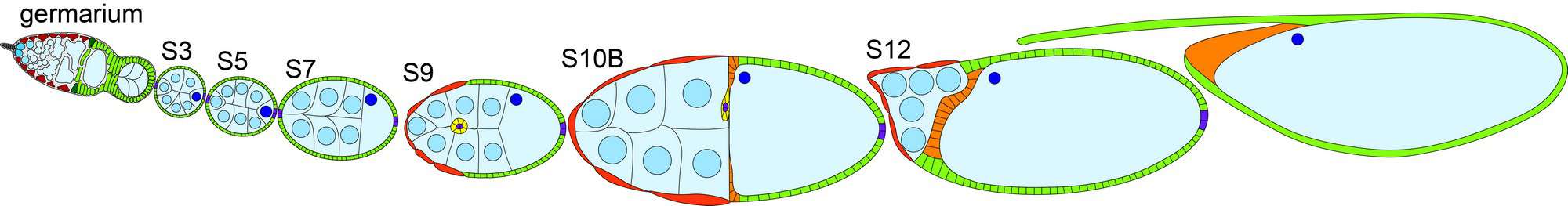
Prostaglandin signaling regulates the actin cytoskeleton
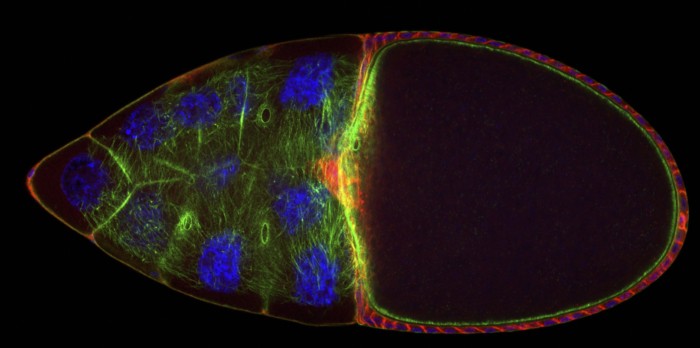
Prostaglandin signaling regulates actin dynamics during follicle development. Both pharmacologic and genetic studies reveal that prostaglandin signaling is required for nurse cell dumping, a process in which the germline derived nurse cells push all of their cytoplasmic contents into the oocyte. In mutants with no prostaglandin signaling the actin structures required for nurse cell dumping are substantially reduced and often completely eliminated. By using a multifaceted experimental approach that combines Drosophila genetics, cell biology, live imaging, and biochemistry we are determining where the prostaglandin signal is coming from, which prostaglandins are involved, whether this is by the canonical signaling pathway, how prostaglandin signaling interacts with known actin regulators, and the downstream changes in gene expression during nurse cell dumping. The results from these studies are likely to provide general insight into how prostaglandins regulate the cytoskeleton at a cellular level. Such mechanisms of prostaglandin action are likely to be reutilized throughout development, including mediating the cytoskeletal changes that occur during cancer progression and metastasis.
Through these studies the lab has identified a number of actin binding proteins as new downstream effectors of prostaglandin signaling. We identified Enabled as an effector of prostaglandins. Our data suggests that prostaglandins inhibit Enabled early in oogenesis to prevent early and aberrant actin filament and aggregate formation. Later, prostaglandins promote Enabled activity to drive bundle formation necessary for nurse cell dumping. We also found that Fascin, an actin bundling protein, is required downstream of prostaglandins to regulate bundle formation necessary for nurse cell dumping and to maintain cortical actin integrity. Importantly, this work provided the first link between prostaglandins and Fascin. Importantly, prostaglandins and the mammalian homologs of Fascin and Enabled (i.e. Mena) are known to play critical roles in cancer development and metastasis. Thus, the same mechanisms by which prostaglandins regulate Drosophila Fascin and Enabled are likely conserved in the instance of cancer. Current efforts are focused on uncover the mechanisms by which prostaglandins regulate these and other actin binding proteins.
In our efforts to determine how prostaglandins regulate Fascin we made the surprising discovery that Fascin is not solely cytoplasmic, but localized to the outside of the nuclear envelope and inside the nucleus. The localization of Fascin to these new cellular sites is conserved from flies to humans. As Fascin is a critical contributor to cancer, understanding these novel Fascin activities is critically important. At the nuclear periphery, Fascin interacts with the Linker of the Nucleoskeleton and Cytoskeleton (LINC) Complex to mediate force transmission to the nucleus. This activity is critical for invasive migration. Inside the nucleus, Fascin regulates nucleolar structure and as discussed below, nuclear actin. Fascin’s localization to both the nuclear periphery and the nucleus, and its nucleolar activity are regulated by prostaglandin signaling. Current efforts are focused on defining when and where the different Fascin activities are utilized to drive cell migration and morphogenesis.
Invasive, collective cell migration
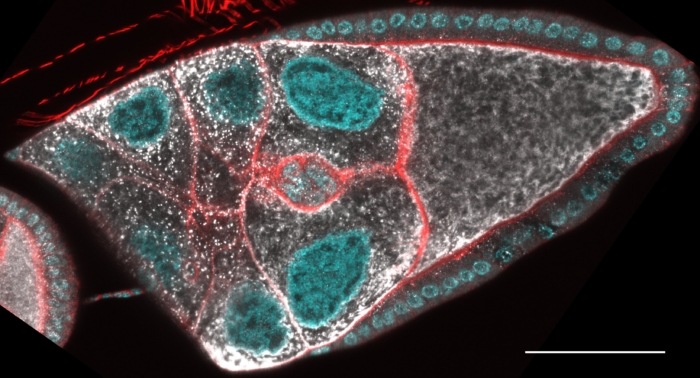
Border cell migration is an excellent model of collective, invasive cell migration. This type of migration is widely observed during development and also mediates cancer metastasis. During Stage 9 of Drosophila oogenesis, 8-10 border cells delaminate from the follicular epithelium and migrate, invasively between the much larger nurse cells to the nurse cell-oocyte boundary.
We are currently exploring the roles of prostaglandin signaling and the three different Fascin activities in border cell migration.
Nuclear actin and actin binding proteins
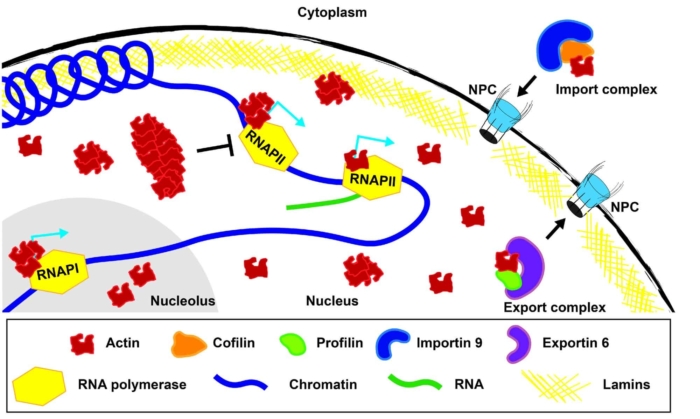 Actin is not solely cytoplasmic but localizes and functions in the nucleus. The nuclear localization of actin is highly regulated. In the cytoplasm actin bind to Cofilin, and this complex is recognized by Importin 9 and translocated to the nucleus. Inside the nucleus, actin-Profilin complexes are recognized by Exportin 6 and taken out of the nucleus. In the nucleus, actin has been implicated in regulating transcription, DNA damage repair, and nuclear structure. However, much remains to be learned including what are the structures of actin in the nucleus, how does the structure impact its function, how is nuclear actin regulated, and what is it doing during development.
Actin is not solely cytoplasmic but localizes and functions in the nucleus. The nuclear localization of actin is highly regulated. In the cytoplasm actin bind to Cofilin, and this complex is recognized by Importin 9 and translocated to the nucleus. Inside the nucleus, actin-Profilin complexes are recognized by Exportin 6 and taken out of the nucleus. In the nucleus, actin has been implicated in regulating transcription, DNA damage repair, and nuclear structure. However, much remains to be learned including what are the structures of actin in the nucleus, how does the structure impact its function, how is nuclear actin regulated, and what is it doing during development.
We have identified multiple reagents that allow us to examine nuclear actin during Drosophila oogenesis. Through characterization of the subnuclear localization, cell-type specificity, and developmental timing of these different actin pools we are developing testable hypotheses for the functions of nuclear actin during follicle development. Additionally, we have uncovered that Fascin regulates nuclear actin, and are currently pursuing the role of prostaglandins in modulating nuclear actin.